We are hiring! Check out our open positions.
We are hiring! Check out our open positions.
Every year, 2,3 million people are diagnosed with breast cancer worldwide, leading to ~670 000 deaths annually¹
After initial treatment, approximately 15% of breast cancer survivors will develop a second breast malignancy within 10 years²
About 70% of patients, diagnosed with the most common form of breast cancer, can safely omit chemotherapy when their recurrence-risk is adequately assessed³
These tests often consume scarce tissue samples, which are limited in quantity. This can be
particularly problematic, as it leaves less material available for other necessary diagnostic
tests, potentially impacting overall patient care.
Our test provides precise prognostic insights, assessing the risk of recurrence and evaluating adjuvant chemotherapy benefits directly from standard H&E slides.
By aiming to reduce overtreatment, potentially lowering costs, and delivering rapid results, it supports healthcare providers in making informed decisions more quickly than standard genomic assays.
*Available for research use only.
Our test is applicable to patients with early-stage hormone receptor-positive, HER2-negative breast cancer, serving 70% of breast cancer patients
Using a fully digital workflow, which only requires uploading digitized H&E stained slides to our platform, we can deliver results on the same day
Our test utilized digitized H&E stained slides, eliminating the need to send over scarce tissue samples
Our digital workflow provides treating physicians with rapid, actionable insights, enhancing patient treatment and outcomes.
One of the first steps in treating breast cancer involves surgically removing the primary tumor. To gain further insights about the type of tumor, the removed tissue is then sent to a pathologist for further examination.
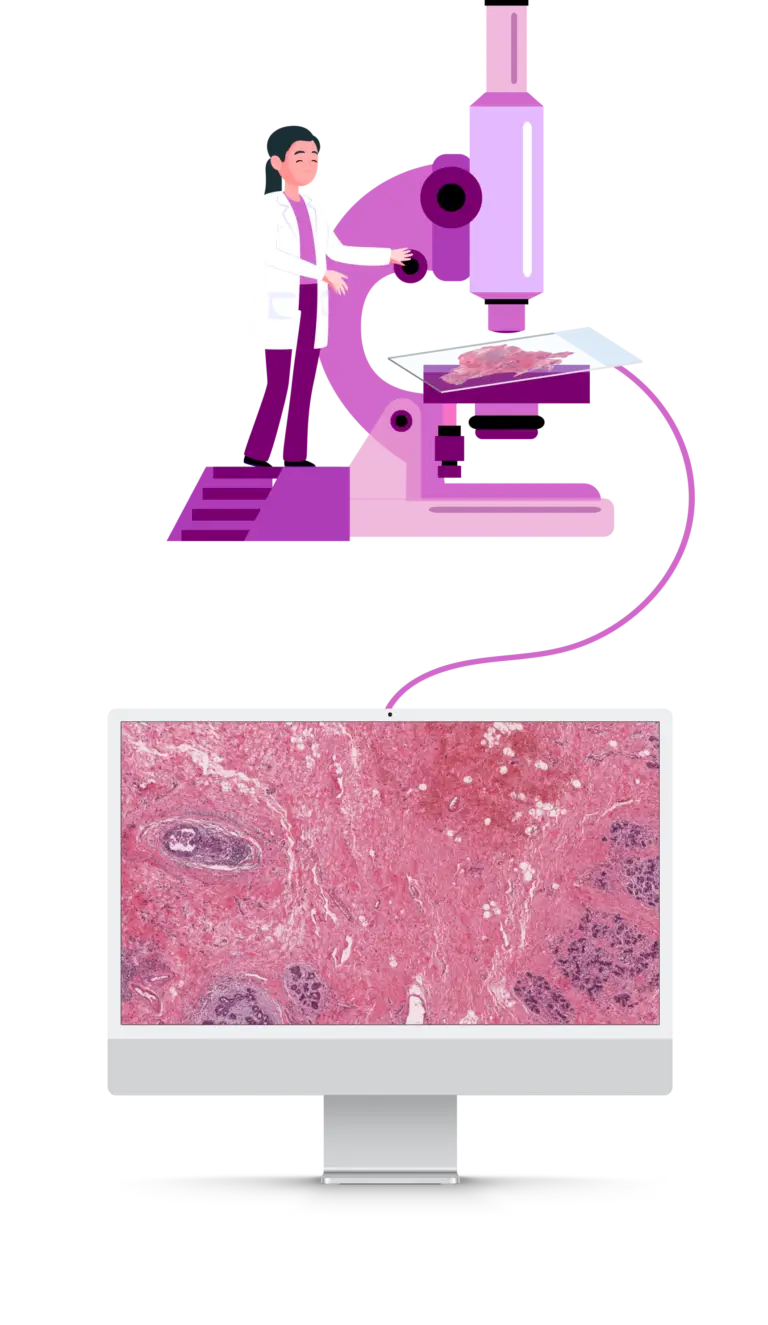
The pathologist prepares the sample for examination under the microscope. To analyze the tissue with our AI models, the microscopic image needs to be digitized. This digitization can be carried out by the pathologist or by our partner network.
The digitized image is uploaded to the StratifAI platform. Our proprietary algorithms analyze the image, assessing various patterns that indicate the recurrence risk of breast cancer. This step leverages artificial intelligence to provide a comprehensive prognostic evaluation.
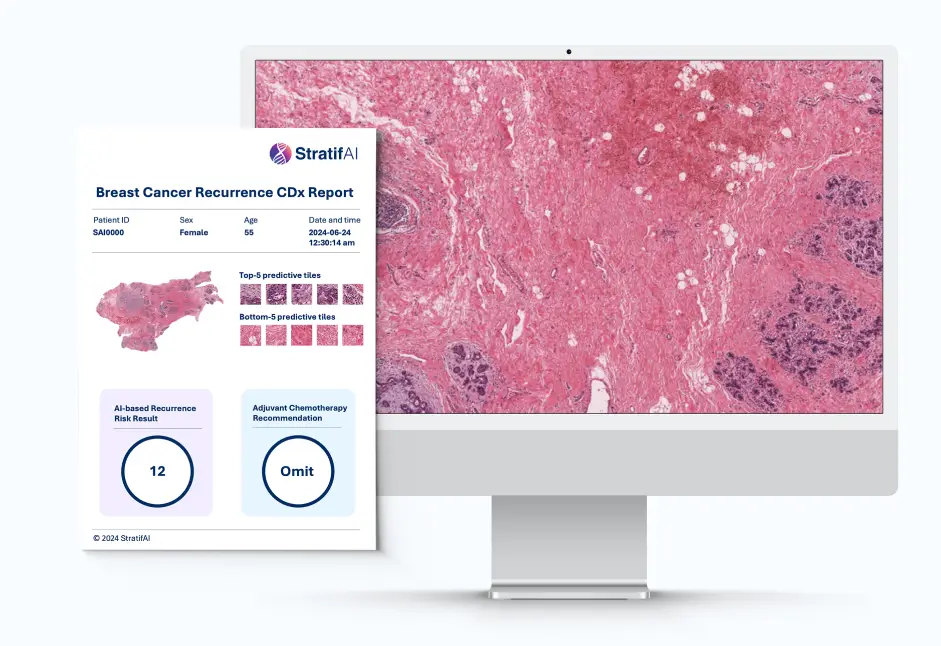
After the analysis is complete, our platform generates a detailed PDF report within seconds. This report includes a recurrence risk score and other relevant insights derived from the image analysis. The PDF is easily accessible and can be shared with healthcare providers to aid in clinical decision-making.
*Research Use Only

Based on the insights provided in the PDF report, healthcare professionals may make informed decisions on whether chemotherapy is necessary. Patients with a high risk score might be recommended to start chemotherapy right away without having to wait for the results from the molecular tests, while patients with a low risk score might be advised to omit chemotherapy. For patients with intermediate risk, chemotherapy treatment may be deferred until further confirmation.
*This product is not approved as a medical device and is available for research use only.
© 2025 StratifAI